A fascinating metal, although not found in many coins
Bullion
Traditionally, silver, and gold coins have been used as a store of wealth. With the value being in the metal itself, any object made of gold, for instance, tends to be valuable. Coins are convenient in that they are solid, compact ways to store that metal. But coins can only be made by certain authorities. An example is this UK 1oz silver bullion coin.
Before being made into coins, lumps of gold and other precious metal are called bullion. The word “bullion” comes from the Anglo-Norman term for a melting-house where metal was refined, and before this the French word bouillon, which in English means “boiling”. The Oxford English dictionary has more information as well. When cast in a mould, bullion (or non-precious metal) is called an “ingot“. Ingots can be any shape, although squares and rectangles are common. Rectangle ingots are also commonly called bars. Regular-sized bars enable stacking of larger ingots (e.g. 1kg). This video on the Perth Mint shows some scenes of bulk ingots of gold at various points. Note that like bullion coins, minted bars are becoming popular. These are very precise in their dimensions, and often sold retail in tamper-proof packaging with additional security features.
The number of gold ingots I have is zero. This would not make for an engaging photograph to accompany this text. I do, however, have an ingot, and it is an interesting metal: Bismuth.
Bismuth
Bismuth gets its name from the Latin word bisemutum, and the German word “wissmuth” meaning white mass. Often confused with tin and lead due to its resemblance to those elements. Bismuth was utilised in ancient times by the Egyptians, Greeks, Asians, Romans and Chinese.
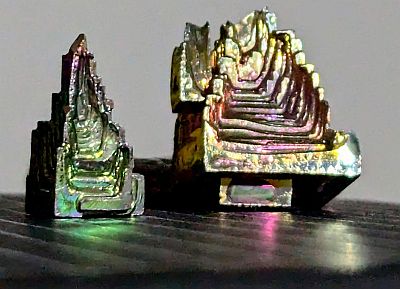
Bismuth Crystals
I had previously seen Bismuth Crystals. These are really cool looking crystals which form in square, pyramid and stepped shapes:
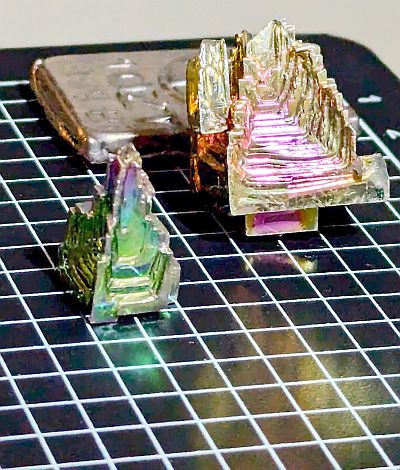
When Bismuth is melted (at 271C / 520F), and allowed to cool naturally, it forms these amazing crystal structures. These can be readily bought and make fantastic decorations. I have these two on my desk.
As a “healing” crystal, Bismuth has been used for centuries to help with a variety of issues, including physical pain, mental stress, and spiritual growth. Bismuth is a stone of transformation. It helps to release negativity and promote positive change. It can also be used to balance emotions, ease anxiety, and promote relaxation.
The art of alchemy was handed down through the centuries from Egypt and Arabia to Greece and Rome, and finally to western and central Europe. The aims of the alchemists were threefold: to find the Stone of Knowledge (The Philosophers’ Stone), to discover the medium of Eternal Youth and Health, and to discover the transmutation of metals.

Bismuth is commonly produced as a by-product from lead and copper smelting. Not quite lead into gold, but Glenn T. Seaborg took this one step further. He successfully transmuted bismuth into gold in 1980. There is hope for my ingot yet!
Bismuth ingots

Like most precious and semi-precious metals, bismuth is available in ingot form. My ingot has the Grimm Metals logo (though I’ve also seen these sold branded as “SPM”), the weight “1 Oz T”, and the fineness of Bismuth, “.999 Bi”. 1 Troy ounce is equal to 31.1035 grams. Note that precious metals are weighed in Troy ounces. This is slightly different to the “avoirdupois” system (used by the UK prior to decimalisation, and still today in the USA). 1-ounce avoirdupois weighs 28.3495 grams.

Unlike minted bars or coins, which are made to very precise dimensions, hand-poured ingots like this are often a little “rougher”. Looking at the edge of mine there are pour lines and gaps where the metal has contracted from the mould when it has cooled.
Radioactivity
Only confirmed recently, Bismuth is actually radioactive. Radioactivity is a natural phenomenon that occurs when the nucleus of an atom spontaneously emits radiation. Half-life is defined as the time it takes for one-half of a radioactive element to decay into a daughter isotope. As radioactive isotopes of elements decay, they lose their radioactivity and become a brand-new element known as a daughter isotope. This can be as little as 1.8 milliseconds for synthetic elements like Oganesson, up to billions of years. In the case of Uranium-235 such as used in Nuclear reactors, the half-life is 700 million years.
Back to Bismuth, it was only discovered to be radioactive in 2003. Scientists calculated its half-life to be 1.9×10^19 years. That is a billion times longer than the age of the universe. Even if all the stars in the universe were to burn out, which would take around 10^14 years based on our understanding of astrophysics, it would still take a hundred thousand times longer for half of the bismuth atoms in a chunk of bismuth to decay. Therefore, bismuth is considered to be ‘barely’ radioactive and safe for everyday use. Ironically, while there is a great deal of discussion about bismuth’s radioactivity, it is being used in radiation shields for CT scans.
In fact, Bismuth is not the only commonly found item which is actually radioactive. For instance, the common banana. Bananas contain a potassium isotope which decays with a half-life of about 1.25 billion years (4×1016 seconds). Only 0.012% of the potassium in a banana is radioactive. You would need to eat 1,000 bananas to result in the same exposure as a chest X-ray. In fact, the amount of radiation in a banana is far less than the regular background radiation you are exposed to just by being on Earth.
Use in numismatics
Finally, back to numismatics, and bismuth is not the most popular metal for coin production. Numista does not list it at all. I wondered if you could get a coin in Bismuth. I found my way to Element Sales where you can get coins in many elements, even gas elements in glass encapsulated coins. (Not specifically a promo, I haven’t bought from them, though they do have Bismuth coins. If you do buy from them, please do let me know what you think of their coins!).
Bismuth is used in pewter. Pewter is a malleable metal alloy. Traditionally it is made from 85–95% tin, mixed with approximately 5-10% antimony, 2% copper, bismuth. Currently, copper and antimony both act as hardeners. In the past, lead was used as a hardener. However, lead began to be removed from the production of the alloy in the early 20th century due to high levels of toxicity discovered in the metal. Subsequently, it was completely removed from pewter production in 1974. Bismuth has increasingly been used to replace lead in Pewter.

Here is a German baptism medal made of pewter. What is the most interesting metal in your collection? Let us know!


Leave a Reply